Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF, /vɛdʒˈɛf/), originally known as vascular permeability factor (VPF),[1] is a signal protein produced by many cells that stimulates the formation of blood vessels. To be specific, VEGF is a sub-family of growth factors, the platelet-derived growth factor family of cystine-knot growth factors. They are important signaling proteins involved in both vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature).
It is part of the system that restores the oxygen supply to tissues when blood circulation is inadequate such as in hypoxic conditions.[2] Serum concentration of VEGF is high in bronchial asthma and diabetes mellitus.[3] VEGF's normal function is to create new blood vessels during embryonic development, new blood vessels after injury, muscle following exercise, and new vessels (collateral circulation) to bypass blocked vessels. It can contribute to disease. Solid cancers cannot grow beyond a limited size without an adequate blood supply; cancers that can express VEGF are able to grow and metastasize. Overexpression of VEGF can cause vascular disease in the retina of the eye and other parts of the body. Drugs such as aflibercept, bevacizumab, ranibizumab, and pegaptanib can inhibit VEGF and control or slow those diseases.
History
[edit]In 1970, Judah Folkman et al. described a factor secreted by tumors causing angiogenesis and called it tumor angiogenesis factor.[4] In 1983 Senger et al. identified a vascular permeability factor secreted by tumors in guinea pigs and hamsters.[1] In 1989 Ferrara and Henzel described an identical factor in bovine pituitary follicular cells which they purified, cloned and named VEGF.[5] A similar VEGF alternative splicing was discovered by Tischer et al. in 1991.[6] Between 1996 and 1997, Christinger and De Vos obtained the crystal structure of VEGF, first at 2.5 Å resolution and later at 1.9 Å.[7][8][9]
Fms-like tyrosine kinase-1 (flt-1) was shown to be a VEGF receptor by Ferrara et al. in 1992.[10] The kinase insert domain receptor (KDR) was shown to be a VEGF receptor by Terman et al. in 1992 as well.[11] In 1998, neuropilin 1 and neuropilin 2 were shown to act as VEGF receptors.[12]
Classification
[edit]
In mammals, the VEGF family comprises five members: VEGF-A, placenta growth factor (PGF), VEGF-B, VEGF-C and VEGF-D. The latter members were discovered after VEGF-A; before their discovery, VEGF-A was known as VEGF. A number of VEGF-related proteins encoded by viruses (VEGF-E) and in the venom of some snakes (VEGF-F) have also been discovered.
| Type | Function |
|---|---|
| VEGF-A |
|
| VEGF-B | Embryonic angiogenesis (myocardial tissue, to be specific)[16] |
| VEGF-C | Lymphangiogenesis[17] |
| VEGF-D | Needed for the development of lymphatic vasculature surrounding lung bronchioles [citation needed] |
| PlGF | Important for Vasculogenesis, Also needed for angiogenesis during ischemia, inflammation, wound healing, and cancer. [citation needed] |
Activity of VEGF-A, as its name implies, has been studied mostly on cells of the vascular endothelium, although it does have effects on a number of other cell types (e.g., stimulation monocyte/macrophage migration, neurons, cancer cells, kidney epithelial cells). In vitro, VEGF-A has been shown to stimulate endothelial cell mitogenesis and cell migration. VEGF-A is also a vasodilator and increases microvascular permeability and was originally referred to as vascular permeability factor.
Isoforms
[edit]
There are multiple isoforms of VEGF-A that result from alternative splicing of mRNA from a single, 8-exon VEGFA gene. These are classified into two groups which are referred to according to their terminal exon (exon 8) splice site: the proximal splice site (denoted VEGFxxx) or distal splice site (VEGFxxxb). In addition, alternate splicing of exon 6 and 7 alters their heparin-binding affinity and amino acid number (in humans: VEGF121, VEGF121b, VEGF145, VEGF165, VEGF165b, VEGF189, VEGF206; the rodent orthologs of these proteins contain one fewer amino acids). These domains have important functional consequences for the VEGF splice variants, as the terminal (exon 8) splice site determines whether the proteins are pro-angiogenic (proximal splice site, expressed during angiogenesis) or anti-angiogenic (distal splice site, expressed in normal tissues). In addition, inclusion or exclusion of exons 6 and 7 mediate interactions with heparan sulfate proteoglycans (HSPGs) and neuropilin co-receptors on the cell surface, enhancing their ability to bind and activate the VEGF receptors (VEGFRs).[18] Recently, VEGF-C has been shown to be an important inducer of neurogenesis in the murine subventricular zone, without exerting angiogenic effects.[19]
Mechanism
[edit]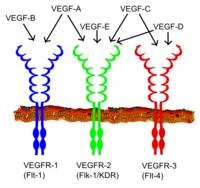
All members of the VEGF family stimulate cellular responses by binding to tyrosine kinase receptors (the VEGFRs) on the cell surface, causing them to dimerize and become activated through transphosphorylation, although to different sites, times, and extents. The VEGF receptors have an extracellular portion consisting of 7 immunoglobulin-like domains, a single transmembrane spanning region, and an intracellular portion containing a split tyrosine-kinase domain. VEGF-A binds to VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1).[21] VEGFR-2 appears to mediate almost all of the known cellular responses to VEGF. The function of VEGFR-1 is less well-defined, although it is thought to modulate VEGFR-2 signaling.[22] Another function of VEGFR-1 may be to act as a dummy/decoy receptor, sequestering VEGF from VEGFR-2 binding (this appears to be particularly important during vasculogenesis in the embryo). VEGF-C and VEGF-D, but not VEGF-A, are ligands for a third receptor (VEGFR-3/Flt4), which mediates lymphangiogenesis. The receptor (VEGFR3) is the site of binding of main ligands (VEGFC and VEGFD), which mediates perpetual action and function of ligands on target cells. Vascular endothelial growth factor-C can stimulate lymphangiogenesis (via VEGFR3) and angiogenesis via VEGFR2. Vascular endothelial growth factor-R3 has been detected in lymphatic endothelial cells in CL of many species, cattle, buffalo and primate.[23]
In addition to binding to VEGFRs, VEGF binds to receptor complexes consisting of both neuropilins and VEGFRs. This receptor complex has increased VEGF signalling activity in endothelial cells (blood vessels).[12][24] Neuropilins (NRP) are pleiotropic receptors and therefore other molecules may interfere with the signalling of the NRP/VEGFR receptor complexes. For example, Class 3 semaphorins compete with VEGF165 for NRP binding and could therefore regulate VEGF-mediated angiogenesis.[25]
Expression
[edit]VEGF-A production can be induced in a cell that is not receiving enough oxygen.[21] When a cell is deficient in oxygen, it produces HIF, hypoxia-inducible factor, a transcription factor. HIF stimulates the release of VEGF-A, among other functions (including modulation of erythropoiesis). Circulating VEGF-A then binds to VEGF receptors on endothelial cells, triggering a tyrosine kinase pathway leading to angiogenesis.[clarification needed] The expression of angiopoietin-2 in the absence of VEGF leads to endothelial cell death and vascular regression.[26] Conversely, a German study done in vivo found that VEGF concentrations actually decreased after a 25% reduction in oxygen intake for 30 minutes.[27] HIF1 alpha and HIF1 beta are constantly being produced but HIF1 alpha is highly O2 labile, so, in aerobic conditions, it is degraded. When the cell becomes hypoxic, HIF1 alpha persists and the HIF1alpha/beta complex stimulates VEGF release. the combined use of microvesicles and 5-FU resulted in enhanced chemosensitivity of squamous cell carcinoma cells more than the use of either 5-FU or microvesicle alone. In addition, down regulation of VEGF gene expression was associated with decreased CD1 gene expression.[28]
Clinical significance
[edit]In disease
[edit]VEGF-A and the corresponding receptors are rapidly up-regulated after traumatic injury of the central nervous system (CNS). VEGF-A is highly expressed in the acute and sub-acute stages of CNS injury, but the protein expression declines over time. This time-span of VEGF-A expression corresponds with the endogenous re-vascularization capacity after injury.[25] This would suggest that VEGF-A / VEGF165 could be used as target to promote angiogenesis after traumatic CNS injuries. However, there are contradicting scientific reports about the effects of VEGF-A treatments in CNS injury models.[25]
Although it has not been associated as a biomarker for the diagnosis of acute ischemic stroke,[29] high levels of serum VEGF in the first 48 hours after an cerebral infarct have been associated with a poor prognosis after 6 months[30] and 2 years.[31]
VEGF-A has been implicated with poor prognosis in breast cancer. Numerous studies show a decreased overall survival and disease-free survival in those tumors overexpressing VEGF. The overexpression of VEGF-A may be an early step in the process of metastasis, a step that is involved in the "angiogenic" switch. Although VEGF-A has been correlated with poor survival, its exact mechanism of action in the progression of tumors remains unclear.[32]
VEGF-A is also released in rheumatoid arthritis in response to TNF-α, increasing endothelial permeability and swelling and also stimulating angiogenesis (formation of capillaries).[33]
VEGF-A is also important in diabetic retinopathy (DR). The microcirculatory problems in the retina of people with diabetes can cause retinal ischaemia, which results in the release of VEGF-A, and a switch in the balance of pro-angiogenic VEGFxxx isoforms over the normally expressed VEGFxxxb isoforms. VEGFxxx may then cause the creation of new blood vessels in the retina and elsewhere in the eye, heralding changes that may threaten the sight.
VEGF-A plays a role in the disease pathology of the wet form age-related macular degeneration (AMD), which is the leading cause of blindness for the elderly of the industrialized world. The vascular pathology of AMD shares certain similarities with diabetic retinopathy, although the cause of disease and the typical source of neovascularization differs between the two diseases.
VEGF-D serum levels are significantly elevated in patients with angiosarcoma.[34]
Once released, VEGF-A may elicit several responses. It may cause a cell to survive, move, or further differentiate. Hence, VEGF is a potential target for the treatment of cancer. The first anti-VEGF drug, a monoclonal antibody named bevacizumab, was approved in 2004. Approximately 10–15% of patients benefit from bevacizumab therapy; however, biomarkers for bevacizumab efficacy are not yet known.
Current studies show that VEGFs are not the only promoters of angiogenesis. In particular, FGF2 and HGF are potent angiogenic factors.
Patients suffering from pulmonary emphysema have been found to have decreased levels of VEGF in the pulmonary arteries.
VEGF-D has also been shown to be over expressed in lymphangioleiomyomatosis and is currently used as a diagnostic biomarker in the treatment of this rare disease.[35]
In the kidney, increased expression of VEGF-A in glomeruli directly causes the glomerular hypertrophy that is associated with proteinuria.[36]
VEGF alterations can be predictive of early-onset pre-eclampsia.[37]
Gene therapies for refractory angina establish expression of VEGF in epicardial cells to promote angiogenesis.[38]
See also
[edit]- Proteases in angiogenesis
- Withaferin A, a potent inhibitor of angiogenesis
References
[edit]- ^ a b Senger, D.; Galli, S.; Dvorak, A.; Perruzzi, C.; Harvey, V.; Dvorak, H. (25 February 1983). "Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid". Science. 219 (4587): 983–985. Bibcode:1983Sci...219..983S. doi:10.1126/science.6823562. PMID 6823562.
- ^ Palmer, Biff F.; Clegg, Deborah J. (2014). "Oxygen sensing and metabolic homeostasis". Molecular and Cellular Endocrinology. 397 (1–2): 51–57. doi:10.1016/j.mce.2014.08.001. PMID 25132648. S2CID 5165215.
- ^ Cooper, Mark; Vranes, Dimitria; Youssef, Sherif; Stacker, Steven A.; Cox, Alison J.; Rizkalla, Bishoy; Casley, David J.; Bach, Leon A.; Kelly, Darren J.; Gilbert, Richard E. (November 1999). "Increased Renal Expression of Vascular Endothelial Growth Factor (VEGF) and Its Receptor VEGFR-2 in Experimental Diabetes". Diabetes. 48 (11): 2229–2239. doi:10.2337/diabetes.48.11.2229. PMID 10535459.
- ^ Folkman, J (1 February 1971). "Isolation of a tumor factor responsible for angiogenesis". Journal of Experimental Medicine. 133 (2): 275–288. doi:10.1084/jem.133.2.275. PMC 2138906. PMID 4332371.
- ^ Ferrara, N; Henzel, WJ (15 June 1989). "Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells". Biochemical and Biophysical Research Communications. 161 (2): 851–858. doi:10.1016/0006-291x(89)92678-8. PMID 2735925.
- ^ Tischer, E; Mitchell, R; Hartman, T; Silva, M; Gospodarowicz, D; Fiddes, JC; Abraham, JA (25 June 1991). "The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing". The Journal of Biological Chemistry. 266 (18): 11947–54. doi:10.1016/S0021-9258(18)99049-6. PMID 1711045.
- ^ Christinger, Hans W.; Muller, Yves A.; Berleau, Lea T.; Keyt, Bruce A.; Cunningham, Brian C.; Ferrara, Napoleone; de Vos, Abraham M. (November 1996). "Crystallization of the receptor binding domain of vascular endothelial growth factor". Proteins: Structure, Function, and Genetics. 26 (3): 353–357. doi:10.1002/(SICI)1097-0134(199611)26:3<353::AID-PROT9>3.0.CO;2-E. PMID 8953654. S2CID 35946525.
- ^ Muller, Yves A.; Li, Bing; Christinger, Hans W.; Wells, James A.; Cunningham, Brian C.; Vos, Abraham M. de (8 July 1997). "Vascular endothelial growth factor: Crystal structure and functional mapping of the kinase domain receptor binding site". Proceedings of the National Academy of Sciences. 94 (14): 7192–7197. Bibcode:1997PNAS...94.7192M. doi:10.1073/pnas.94.14.7192. PMC 23789. PMID 9207067.
- ^ Muller, Yves A; Christinger, Hans W; Keyt, Bruce A; de Vos, Abraham M (October 1997). "The crystal structure of vascular endothelial growth factor (VEGF) refined to 1.93 Å resolution: multiple copy flexibility and receptor binding". Structure. 5 (10): 1325–1338. doi:10.1016/s0969-2126(97)00284-0. PMID 9351807.
- ^ Vries, C. de; Escobedo, J. A.; Ueno, H.; Houck, K.; Ferrara, N.; Williams, L. T. (21 February 1992). "The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor". Science. 255 (5047): 989–991. Bibcode:1992Sci...255..989D. doi:10.1126/science.1312256. PMID 1312256.
- ^ Terman, Bruce I.; Dougher-Vermazen, Maureen; Carrion, Miguel E.; Dimitrov, Dragan; Armellino, Douglas C.; Gospodarowicz, Denis; Böhlen, Peter (30 September 1992). "Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor". Biochemical and Biophysical Research Communications. 187 (3): 1579–1586. doi:10.1016/0006-291x(92)90483-2. PMID 1417831.
- ^ a b Soker, Shay; Takashima, Seiji; Miao, Hua Quan; Neufeld, Gera; Klagsbrun, Michael (March 1998). "Neuropilin-1 Is Expressed by Endothelial and Tumor Cells as an Isoform-Specific Receptor for Vascular Endothelial Growth Factor". Cell. 92 (6): 735–745. doi:10.1016/s0092-8674(00)81402-6. PMID 9529250. S2CID 547080.
- ^ Bang, Seokyoung; Lee, Seung-Ryeol; Ko, Jihoon; Son, Kyungmin; Tahk, Dongha; Ahn, Jungho; Im, Changkyun; LiJeon, Noo (14 August 2017). "A Low Permeability Microfluidic Blood-Brain Barrier Platform with Direct Contact between Perfusable Vascular Network and Astrocytest". Scientific Reports. 7 (1): 8083. Bibcode:2017NatSR...7.8083B. doi:10.1038/s41598-017-07416-0. PMC 5556097. PMID 28808270.
- ^ Ivet Elias; Sylvie Franckhauser; Fatima Bosch (1 April 2013). "New insights into adipose tissue VEGF-A actions in the control of obesity and insulin resistance". Adipocyte. 2 (2): 109–112. doi:10.4161/adip.22880. PMC 3661112. PMID 23805408.
- ^ Cursiefen, Claus; Chen, Lu; Borges, Leonardo P.; Jackson, David; Cao, Jingtai; Radziejewski, Czeslaw; D’Amore, Patricia A.; Dana, M. Reza; Wiegand, Stanley J.; Streilein, J. Wayne (1 April 2004). "VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment". Journal of Clinical Investigation. 113 (7): 1040–1050. doi:10.1172/JCI200420465. PMC 379325. PMID 15057311.
- ^ Claesson-Welsh, L. (20 August 2008). "VEGF-B Taken to Our Hearts: Specific Effect of VEGF-B in Myocardial Ischemia". Arteriosclerosis, Thrombosis, and Vascular Biology. 28 (9): 1575–1576. doi:10.1161/ATVBAHA.108.170878. PMID 18716319.
- ^ Mandriota, S. J.; Jussila, L.; Jeltsch, M.; Compagni, A.; Baetens, D.; Prevo, R.; Banerji, S.; Huarte, J.; Montesano, R.; Jackson, D. G.; Orci, L.; Alitalo, K.; Christofori, G.; Pepper, M. S. (15 February 2001). "Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis". The EMBO Journal. 20 (4): 672–682. doi:10.1093/emboj/20.4.672. PMC 145430. PMID 11179212. Retrieved 3 February 2022.
- ^ Cébe Suarez, S.; Pieren, M.; Cariolato, L.; Arn, S.; Hoffmann, U.; Bogucki, A.; Manlius, C.; Wood, J.; Ballmer-Hofer, K. (September 2006). "A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2". Cellular and Molecular Life Sciences. 63 (17): 2067–2077. doi:10.1007/s00018-006-6254-9. PMC 11136335. PMID 16909199. S2CID 28267679.
- ^ Shin, Y. J.; Choi, J. S.; et al. (2010). "Induction of vascular endothelial growth factor receptor-3 mRNA in glial cells following focal cerebral ischemia in rats". J Neuroimmunol. 229 (1–2): 81–90. doi:10.1016/j.jneuroim.2010.07.008. PMID 20692049. S2CID 21073290.
- ^ Häggström, Mikael (2014). "Medical gallery of Mikael Häggström 2014". WikiJournal of Medicine. 1 (2). doi:10.15347/wjm/2014.008.
- ^ a b Holmes, Katherine; Roberts, Owain Ll; Thomas, Angharad M.; Cross, Michael J. (2007). "Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition". Cellular Signalling. 19 (10): 2003–12. doi:10.1016/j.cellsig.2007.05.013. PMID 17658244.
- ^ Karkkainen, M.J.; Petrova, T.V. (2000). "Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis". Oncogene. 19 (49): 5598–5605. doi:10.1038/sj.onc.1203855. PMID 11114740. S2CID 2374117.
- ^ Ali, Ibne; et al. (2013). "Expression and localization of locally produced growth factors regulating lymphangiogenesis during different stages of the estrous cycle in corpus luteum of buffalo" (Bubalus bubalis)". Theriogenology. 81 (3): 428–436. doi:10.1016/j.theriogenology.2013.10.017. PMID 24246422.
- ^ Herzog, Birger; Pellet-Many, Caroline; Britton, Gary; Hartzoulakis, Basil; Zachary, Ian C. (8 June 2011). "VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation". Molecular Biology of the Cell. 22 (15): 2766–2776. doi:10.1091/mbc.E09-12-1061. PMC 3145551. PMID 21653826.
- ^ a b c Mecollari, Vasil; Nieuwenhuis, Bart; Verhaagen, Joost (27 October 2014). "A perspective on the role of class III semaphorin signaling in central nervous system trauma". Frontiers in Cellular Neuroscience. 8: 328. doi:10.3389/fncel.2014.00328. PMC 4209881. PMID 25386118.
- ^ Harmey, Judith (2004). VEGF and cancer. Georgetown, Tex: Landes Bioscience/Eurekah.com New York, N.Y. Kluwer Academic/Plenum Publishers. ISBN 978-0-306-47988-5.[page needed]
- ^ Oltmanns, Kerstin M.; Gehring, Hartmut; Rudolf, Sebastian; Schultes, Bernd; Hackenberg, Claudia; Schweiger, Ulrich; Born, Jan; Fehm, Horst L.; Peters, Achim (1 March 2006). "Acute hypoxia decreases plasma VEGF concentration in healthy humans". American Journal of Physiology. Endocrinology and Metabolism. 290 (3): E434–E439. doi:10.1152/ajpendo.00508.2004. PMID 16219663. S2CID 32679788.
- ^ Abd El Latif, Ghada; Aboushady, Iman; Sabry, Dina (1 April 2019). "Decreased VEGF and Cyclin D1 Genes Expression Enhances Chemosensitivity of Human Squamous Cell Carcinoma Cells to 5-Fluorouracil and/or Mesenchymal Stem Cells-Derived Microvesicles". Egyptian Dental Journal. 65 (2): 1217–1228. doi:10.21608/EDJ.2019.72197.
- ^ Seidkhani-Nahal, Ali; Khosravi, Afra; Mirzaei, Asad; Basati, Gholam; Abbasi, Milad; Noori-Zadeh, Ali (5 September 2020). "Serum vascular endothelial growth factor (VEGF) levels in ischemic stroke patients: a systematic review and meta-analysis of case–control studies". Neurological Sciences. 42 (5): 1811–1820. doi:10.1007/s10072-020-04698-7. PMID 32888077. S2CID 221494935.
- ^ Escudero, Carlos; Acurio, Jesenia; López, Eduardo; Rodríguez, Andrés; Benavente, Antonia; Lara, Evelyn; Korzeniewski, Steven J. (2020). "Vascular endothelial growth factor and poor prognosis after ischaemic stroke". European Journal of Neurology. 28 (5): 1759–1764. doi:10.1111/ene.14641. PMID 33176035. S2CID 226310802.
- ^ Åberg, N. David; Wall, Alexander; Anger, Olof; Jood, Katarina; Andreasson, Ulf; Blennow, Kaj; Zetterberg, Henrik; Isgaard, Jörgen; Jern, Christina; Svensson, Johan (May 2020). "Circulating levels of vascular endothelial growth factor and post-stroke long-term functional outcome". Acta Neurologica Scandinavica. 141 (5): 405–414. doi:10.1111/ane.13219. PMID 31919840.
- ^ Mohammed, R. a. A.; Green, A.; El-Shikh, S.; Paish, E. C.; Ellis, I. O.; Martin, S. G. (April 2007). "Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis". British Journal of Cancer. 96 (7): 1092–1100. doi:10.1038/sj.bjc.6603678. ISSN 1532-1827. PMC 2360132. PMID 17353919.
- ^ Taylor, Peter C. (2002). "VEGF and imaging of vessels in rheumatoid arthritis". Arthritis Research. 4 (Suppl 3): S99–107. doi:10.1186/ar582. ISSN 1465-9905. PMC 3240157. PMID 12110128.
- ^ Amo, Y.; Masuzawa, M.; Hamada, Y.; Katsuoka, K. (2004). "Serum concentrations of vascular endothelial growth factor-D in angiosarcoma patients". British Journal of Dermatology. 150 (1): 160–1. doi:10.1111/j.1365-2133.2004.05751.x. PMID 14746640. S2CID 38291933.
- ^ Young, Lisa R.; Inoue, Yoshikazu; McCormack, Francis X. (10 January 2008). "Diagnostic Potential of Serum VEGF-D for Lymphangioleiomyomatosis". New England Journal of Medicine. 358 (2): 199–200. doi:10.1056/NEJMc0707517. PMC 3804557. PMID 18184970.
- ^ Liu, E.; Morimoto, M.; Kitajima, S.; Koike, T.; Yu, Y.; Shiiki, H.; Nagata, M.; Watanabe, T.; Fan, J. (2007). "Increased Expression of Vascular Endothelial Growth Factor in Kidney Leads to Progressive Impairment of Glomerular Functions". Journal of the American Society of Nephrology. 18 (7): 2094–104. doi:10.1681/ASN.2006010075. PMID 17554151.
- ^ Andraweera, P. H.; Dekker, G. A.; Roberts, C. T. (2012). "The vascular endothelial growth factor family in adverse pregnancy outcomes". Human Reproduction Update. 18 (4): 436–457. doi:10.1093/humupd/dms011. PMID 22495259.
- ^ "Gene therapy for refractory angina". Genome Context. 16 October 2019. Archived from the original on 16 October 2019. Retrieved 16 October 2019.
Further reading
[edit]- Bengoetxea H, Argandoña EG, Lafuente JV (2008). "Effects of Visual Experience on Vascular Endothelial Growth Factor Expression during the Postnatal Development of the Rat Visual Cortex". Cerebral Cortex. 18 (7): 1630–39. doi:10.1093/cercor/bhm190. PMC 2430152. PMID 17986606.
- Zan L, Wu H, Jiang J, Zhao S, Song Y, Teng G, Li H, Jia Y, Zhou M, Zhang X, Qi J, Wang J (2011). "Temporal profile of Src, SSeCKS, and angiogenic factors after focal cerebral ischemia: correlations with angiogenesis and cerebral edema". Neurochem. Int. 58 (8): 872–9. doi:10.1016/j.neuint.2011.02.014. PMC 3100427. PMID 21334414.
- Zan L, Zhang X, Xi Y, Wu H, Song Y, Teng G, Li H, Qi J, Wang J (2014). "Src regulates angiogenic factors and vascular permeability after focal cerebral ischemia-reperfusion". Neuroscience. 262: 118–28. doi:10.1016/j.neuroscience.2013.12.060. PMC 3943922. PMID 24412374.
- Wang J, Fu X, Jiang C, Yu L, Wang M, Han W, Liu L, Wang J (2014). "Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the VEGF-VEGFR2 signaling pathway in a rat model of vascular dementia". Behav. Brain Res. 265: 171–80. doi:10.1016/j.bbr.2014.02.033. PMC 4000455. PMID 24589546.
- Ferrara N, Gerber HP (2002). "The role of vascular endothelial growth factor in angiogenesis". Acta Haematol. 106 (4): 148–56. doi:10.1159/000046610. PMID 11815711. S2CID 46785882.
- Orpana A, Salven P (2003). "Angiogenic and lymphangiogenic molecules in hematological malignancies". Leuk. Lymphoma. 43 (2): 219–24. doi:10.1080/10428190290005964. PMID 11999550. S2CID 21908151.
- Afuwape AO, Kiriakidis S, Paleolog EM (2003). "The role of the angiogenic molecule VEGF in the pathogenesis of rheumatoid arthritis". Histol. Histopathol. 17 (3): 961–72. PMID 12168808.
- de Bont ES, Neefjes VM, Rosati S, et al. (2003). "New vessel formation and aberrant VEGF/VEGFR signaling in acute leukemia: does it matter?". Leuk. Lymphoma. 43 (10): 1901–9. doi:10.1080/1042819021000015844. PMID 12481883. S2CID 45095413.
- Ria R, Roccaro AM, Merchionne F, et al. (2003). "Vascular endothelial growth factor and its receptors in multiple myeloma". Leukemia. 17 (10): 1961–6. doi:10.1038/sj.leu.2403076. PMID 14513045. S2CID 2335518.
- Caldwell RB, Bartoli M, Behzadian MA, et al. (2004). "Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives". Diabetes Metab. Res. Rev. 19 (6): 442–55. doi:10.1002/dmrr.415. PMID 14648803. S2CID 24931730.
- Patan, Sybill (2004). "Vasculogenesis and Angiogenesis". Angiogenesis in Brain Tumors. Cancer Treatment and Research. Vol. 117. pp. 3–32. doi:10.1007/978-1-4419-8871-3_1. ISBN 978-1-4613-4699-9. PMID 15015550.
- Machein, Marcia Regina; Plate, Karl Heinz (2004). "Role of VEGF in Developmental Angiogenesis and in Tumor Angiogenesis in the Brain". Angiogenesis in Brain Tumors. Cancer Treatment and Research. Vol. 117. pp. 191–218. doi:10.1007/978-1-4419-8871-3_13. ISBN 978-1-4613-4699-9. PMID 15015562.
- Eremina V, Quaggin SE (2004). "The role of VEGF-A in glomerular development and function". Curr. Opin. Nephrol. Hypertens. 13 (1): 9–15. doi:10.1097/00041552-200401000-00002. PMID 15090854. S2CID 24212588.
- Storkebaum E, Lambrechts D, Carmeliet P (2004). "VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection". BioEssays. 26 (9): 943–54. doi:10.1002/bies.20092. PMID 15351965. S2CID 871954.
- Ribatti D (2005). "The crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: a historical review". Br. J. Haematol. 128 (3): 303–9. doi:10.1111/j.1365-2141.2004.05291.x. PMID 15667531. S2CID 1906340.
- Loureiro RM, D'Amore PA (2005). "Transcriptional regulation of vascular endothelial growth factor in cancer". Cytokine Growth Factor Rev. 16 (1): 77–89. doi:10.1016/j.cytogfr.2005.01.005. PMID 15733833.
- Herbst RS, Onn A, Sandler A (2005). "Angiogenesis and lung cancer: prognostic and therapeutic implications". J. Clin. Oncol. 23 (14): 3243–56. doi:10.1200/JCO.2005.18.853. PMID 15886312.
- Pufe T, Kurz B, Petersen W, et al. (2006). "The influence of biomechanical parameters on the expression of VEGF and endostatin in the bone and joint system". Ann. Anat. 187 (5–6): 461–72. doi:10.1016/j.aanat.2005.06.008. PMID 16320826.
- Tong JP, Yao YF (2006). "Contribution of VEGF and PEDF to choroidal angiogenesis: a need for balanced expressions". Clin. Biochem. 39 (3): 267–76. doi:10.1016/j.clinbiochem.2005.11.013. PMID 16409998.
- Lambrechts D, Carmeliet P (2007). "VEGF at the neurovascular interface: therapeutic implications for motor neuron disease". Biochim. Biophys. Acta. 1762 (11–12): 1109–21. doi:10.1016/j.bbadis.2006.04.005. PMID 16784838.
- Matsumoto T, Mugishima H (2006). "Signal transduction via vascular endothelial growth factor (VEGF) receptors and their roles in atherogenesis". J. Atheroscler. Thromb. 13 (3): 130–5. doi:10.5551/jat.13.130. PMID 16835467.
- Bogaert E, Van Damme P, Van Den Bosch L, Robberecht W (2006). "Vascular endothelial growth factor in amyotrophic lateral sclerosis and other neurodegenerative diseases". Muscle Nerve. 34 (4): 391–405. doi:10.1002/mus.20609. PMID 16856151. S2CID 22086357.
- Mercurio AM, Lipscomb EA, Bachelder RE (2006). "Non-angiogenic functions of VEGF in breast cancer". Journal of Mammary Gland Biology and Neoplasia. 10 (4): 283–90. CiteSeerX 10.1.1.476.2778. doi:10.1007/s10911-006-9001-9. PMID 16924371. S2CID 16565983.
- Makinde T, Murphy RF, Agrawal DK (2007). "Immunomodulatory role of vascular endothelial growth factor and angiopoietin-1 in airway remodeling". Curr. Mol. Med. 6 (8): 831–41. doi:10.2174/156652406779010795. PMID 17168735.
- Rini BI, Rathmell WK (2007). "Biological aspects and binding strategies of vascular endothelial growth factor in renal cell carcinoma". Clin. Cancer Res. 13 (2 Pt 2): 741s–746s. doi:10.1158/1078-0432.CCR-06-2110. PMID 17255303.
- Jiang, Chao; Zuo, Fangfang; Wang, Yuejuan; Lu, Hong; Yang, Qingwu; Wang, Jian (1 January 2017). "Progesterone Changes VEGF and BDNF Expression and Promotes Neurogenesis After Ischemic Stroke". Molecular Neurobiology. 54 (1): 571–581. doi:10.1007/s12035-015-9651-y. PMC 4938789. PMID 26746666.
- Rodgers LS, Lalani S, Hardy KM, Xiang X, Broka D, Antin PB, Camenisch TD (2006). "Depolymerized hyaluronan induces vascular endothelial growth factor, a negative regulator of developmental epithelial-to-mesenchymal transformation". Circ. Res. 99 (6): 583–9. doi:10.1161/01.RES.0000242561.95978.43. PMID 16931798.
- Qaum, T; Xu, Q; Joussen, AM; et al. (2001). "VEGF-initiated blood-retinal barrier breakdown in early diabetes". Invest Ophthalmol Vis Sci. 42 (10): 2408–2413. PMID 11527957.
External links
[edit]- Vascular+Endothelial+Growth+Factors at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Proteopedia Vascular_Endothelial_Growth_Factor – the Vascular Endothelial Growth Factor Structure in Interactive 3D
