Rotaxane
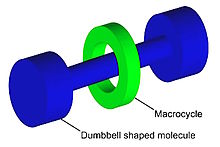

A rotaxane (from Latin rota 'wheel' and axis 'axle') is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle (see graphical representation). The two components of a rotaxane are kinetically trapped since the ends of the dumbbell (often called stoppers) are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bonds.
Much of the research concerning rotaxanes and other mechanically interlocked molecular architectures, such as catenanes, has been focused on their efficient synthesis or their utilization as artificial molecular machines. However, examples of rotaxane substructure have been found in naturally occurring peptides, including: cystine knot peptides, cyclotides or lasso-peptides such as microcin J25.
Synthesis
[edit]The earliest reported synthesis of a rotaxane in 1967 relied on the statistical probability that if two halves of a dumbbell-shaped molecule were reacted in the presence of a macrocycle that some small percentage would connect through the ring.[2] To obtain a reasonable quantity of rotaxane, the macrocycle was attached to a solid-phase support and treated with both halves of the dumbbell 70 times and then severed from the support to give a 6% yield. However, the synthesis of rotaxanes has advanced significantly and efficient yields can be obtained by preorganizing the components utilizing hydrogen bonding, metal coordination, hydrophobic forces, covalent bonds, or coulombic interactions. The three most common strategies to synthesize rotaxane are "capping", "clipping", and "slipping",[3] though others do exist.[4][5] Recently, Leigh and co-workers described a new pathway to mechanically interlocked architectures involving a transition-metal center that can catalyse a reaction through the cavity of a macrocycle.[6]

Capping
[edit]
Synthesis via the capping method relies strongly upon a thermodynamically driven template effect; that is, the "thread" is held within the "macrocycle" by non-covalent interactions, for example rotaxinations with cyclodextrin macrocycles involve exploitation of the hydrophobic effect. This dynamic complex or pseudorotaxane is then converted to the rotaxane by reacting the ends of the threaded guest with large groups, preventing disassociation.[8]
Clipping
[edit]The clipping method is similar to the capping reaction except that in this case the dumbbell shaped molecule is complete and is bound to a partial macrocycle. The partial macrocycle then undergoes a ring closing reaction around the dumbbell-shaped molecule, forming the rotaxane.[9]
Slipping
[edit]The method of slipping is one which exploits the thermodynamic[10] stability of the rotaxane. If the end groups of the dumbbell are an appropriate size it will be able to reversibly thread through the macrocycle at higher temperatures. By cooling the dynamic complex, it becomes kinetically trapped as a rotaxane at the lower temperature.
Snapping
[edit]Snapping involves two separate parts of the thread, both containing a bulky group. one part of the thread is then threaded to the macrocycle, forming a semi rotaxane, and end is closed of by the other part of the thread forming the rotaxane.
"Active template" methodology
[edit]Leigh and co-workers recently began to explore a strategy in which template ions could also play an active role in promoting the crucial final covalent bond forming reaction that captures the interlocked structure (i.e., the metal has a dual function, acting as a template for entwining the precursors and catalyzing covalent bond formation between the reactants).
Potential applications
[edit]
Molecular machines
[edit]Rotaxane-based molecular machines have been of initial interest for their potential use in molecular electronics as logic molecular switching elements and as molecular shuttles.[12][13] These molecular machines are usually based on the movement of the macrocycle on the dumbbell. The macrocycle can rotate around the axis of the dumbbell like a wheel and axle or it can slide along its axis from one site to another. Controlling the position of the macrocycle allows the rotaxane to function as a molecular switch, with each possible location of the macrocycle corresponding to a different state. These rotaxane machines can be manipulated both by chemical [14] and photochemical inputs.[15] Rotaxane based systems have also been shown to function as molecular muscles.[16][17] In 2009, there was a report of a "domino effect" from one extremity to the other in a Glycorotaxane Molecular Machine. In this case, the 4C1 or 1C4 chair-like conformation of the mannopyranoside stopper can be controlled, depending on the localization of the macrocycle.[18] In 2012, unique pseudo-macrocycles consisting of double-lasso molecular machines (also called rotamacrocycles) were reported in Chem. Sci. These structures can be tightened or loosened depending on pH. A controllable jump rope movement was also observed in these new molecular machines.[19]
Ultrastable dyes
[edit]Potential application as long-lasting dyes is based on the enhanced stability of the inner portion of the dumbbell-shaped molecule.[20][21] Studies with cyclodextrin-protected rotaxane azo dyes established this characteristic. More reactive squaraine dyes have also been shown to have enhanced stability by preventing nucleophilic attack of the inner squaraine moiety.[22] The enhanced stability of rotaxane dyes is attributed to the insulating effect of the macrocycle, which is able to block interactions with other molecules.
Nanorecording
[edit]In a nanorecording application,[23] a certain rotaxane is deposited as a Langmuir–Blodgett film on ITO-coated glass. When a positive voltage is applied with the tip of a scanning tunneling microscope probe, the rotaxane rings in the tip area switch to a different part of the dumbbell and the resulting new conformation makes the molecules stick out 0.3 nanometer from the surface. This height difference is sufficient for a memory dot. It is not yet known how to erase such a nanorecording film.
Nomenclature
[edit]Accepted nomenclature is to designate the number of components of the rotaxane in brackets as a prefix.[24] Therefore, the a rotaxane consisting of a single dumbbell-shaped axial molecule with a single macrocycle around its shaft is called a [2]rotaxane, and two cyanostar molecules around the central phosphate group of dialkylphosphate is a [3]rotaxane.
See also
[edit]- Catenane
- Mechanically interlocked molecular architecture
- Molecular Borromean rings
- Molecular knots
- Polyrotaxane
References
[edit]- ^ Bravo, José A.; Raymo, Françisco M.; Stoddart, J. Fraser; White, Andrew J. P.; Williams, David J. (1998). "High Yielding Template-Directed Syntheses of [2]Rotaxanes". Eur. J. Org. Chem. 1998 (11): 2565–2571. doi:10.1002/(SICI)1099-0690(199811)1998:11<2565::AID-EJOC2565>3.0.CO;2-8.
- ^ Harrison, Ian Thomas.; Harrison, Shuyen. (1967). "Synthesis of a stable complex of a macrocycle and a threaded chain". J. Am. Chem. Soc. 89 (22): 5723–5724. doi:10.1021/ja00998a052.
- ^ Aricó, F. (2005). "Templated Synthesis of Interlocked Molecules". Templates in Chemistry II. Vol. 249. pp. 203–259. doi:10.1007/b104330. hdl:10278/33611. ISBN 978-3-540-23087-8.
{{cite book}}:|journal=ignored (help) - ^ Yoon, I; Narita, M; Shimizu, T; Asakawa, M (2004). "Threading-Followed-by-Shrinking Protocol for the Synthesis of a [2]Rotaxane Incorporating a Pd(II)-Salophen Moiety". J. Am. Chem. Soc. 126 (51): 16740–16741. doi:10.1021/ja0464490. PMID 15612709.
- ^ Kameta, N; Hiratani, K; Nagawa, Y (2004). "A novel synthesis of chiral rotaxanes via covalent bond formation". Chem. Commun. (51): 466–467. doi:10.1039/b314744d. PMID 14765261.
- ^ Aucagne, V; Berna, J; Crowley, J. D.; Goldup, S. M.; Hänni, K. D.; Leigh, D. A.; Lusby, P. J.; Ronaldson, V. E.; Slawin, A. M.; Viterisi, A; Walker, D. B. (2007). "Catalytic "active-metal" template synthesis of [2]rotaxanes, [3]rotaxanes, and molecular shuttles, and some observations on the mechanism of the Cu(I)-catalyzed azide-alkyne 1,3-cycloaddition". J. Am. Chem. Soc. 129 (39): 11950–11963. doi:10.1021/ja073513f. PMID 17845039.
- ^ List, Jonathan; Falgenhauer, Elisabeth; Kopperger, Enzo; Pardatscher, Günther; Simmel, Friedrich C. (2016). "Long-range movement of large mechanically interlocked DNA nanostructures". Nature Communications. 7: 12414. Bibcode:2016NatCo...712414L. doi:10.1038/ncomms12414. PMC 4980458. PMID 27492061.
- ^ "Rotaxane by capping". youtube.com. 10 March 2017.
- ^ Romero, Antonio (10 March 2017). "Rotaxane by capping 3d". Rotaxane by capping 3d. 3D video.
- ^ Carson J. Bruns; J. Fraser Stoddart (7 November 2016). The Nature of the Mechanical Bond: From Molecules to Machines. John Wiley & Sons. pp. 271–. ISBN 978-1-119-04400-0.
- ^ Stanier, Carol A.; o'Connell, Michael J.; Anderson, Harry L.; Clegg, William (2001). "Synthesis of fluorescent stilbene and tolan rotaxanes by Suzuki coupling". Chem. Commun. (5): 493–494. doi:10.1039/b010015n.
- ^ Schalley, C. A.; Beizai, K; Vögtle, F (2001). "On the Way to Rotaxane-Based Molecular Motors: Studies in Molecular Mobility and Topological Chirality". Acc. Chem. Res. 34 (6): 465–476. doi:10.1021/ar000179i. PMID 11412083.
- ^ Sauvage, J. P. (1999). "Transition Metal-Containing Rotaxanes and Catenanes in Motion: Toward Molecular Machines and Motors". ChemInform. 30 (4): no. doi:10.1002/chin.199904221.
- ^ Coutrot, F.; Busseron, E. (2008). "A New Glycorotaxane Molecular Machine Based on an Anilinium and a Triazolium Station". Chem. Eur. J. 14 (16): 4784–4787. doi:10.1002/chem.200800480. PMID 18409178.
- ^ Serreli, V; Lee, C. F.; Kay, E. R.; Leigh, D. A. (2007). "Exercising Demons: A Molecular Information Ratchet". Nature. 445 (7127): 523–527. Bibcode:2007Natur.445..523S. doi:10.1038/nature05452. PMID 17268466. S2CID 4314051.
- ^ Coutrot, F; Romuald, C; Busseron, E (2008). "A New pH-Switchable Dimannosyl [c2]Daisy Chain Molecular Machine". Org. Lett. 10 (17): 3741–3744. doi:10.1021/ol801390h. PMID 18666774.
- ^ Radha Kishan, M; Parham, A; Schelhase, F; Yoneva, A; Silva, G; Chen, X; Okamoto, Y; Vögtle, F (2006). "Bridging Rotaxanes' wheels – cyclochiral Bonnanes". Angew. Chem. Int. Ed. 45 (43): 7296–7299. doi:10.1002/anie.200602002. PMID 17029314.
- ^ Coutrot, F.; Busseron, E. (2009). "Controlling the Chair Conformation of a Mannopyranose in a Large-Amplitude [2]Rotaxane Molecular Machine". Chem. Eur. J. 15 (21): 5186–5190. doi:10.1002/chem.200900076. PMID 19229918.
- ^ Romuald, Camille; Ardá, Ana; Clavel, Caroline; Jiménez-Barbero, Jesús; Coutrot, Frédéric (2012). "Tightening or loosening a pH-sensitive double-lasso molecular machine readily synthesized from an ends-activated [c2]daisy chain". Chem. Sci. 3 (6): 1851–1857. doi:10.1039/C2SC20072D. hdl:10261/60415.
- ^ Buston, Jonathan E. H.; Young, James R.; Anderson, Harry L. (2000). "Rotaxane-encapsulated cyanine dyes: enhanced fluorescence efficiency and photostability". Chem. Commun. (11): 905–906. doi:10.1039/b001812k.
- ^ Craig, M. R.; Hutchings, M. G.; Claridge, T. D.; Anderson, H. L. (1998). "Rotaxane-Encapsulation Enhances the Stability of an Azo Dye, in Solution and when Bonded to Cellulose". Angew. Chem. Int. Ed. 40 (6): 1071–1074. doi:10.1002/1521-3773(20010316)40:6<1071::AID-ANIE10710>3.0.CO;2-5. PMID 11268077.
- ^ Arunkumar, E; Forbes, C. C.; Noll, B. C.; Smith, B. D. (2005). "Squaraine-Derived Rotaxanes: Sterically Protected Fluorescent Near-IR Dyes" (PDF). J. Am. Chem. Soc. 127 (10): 3288–3289. doi:10.1021/ja042404n. PMID 15755140. Archived (PDF) from the original on 2022-10-09.
- ^ Feng, M; Guo, X; Lin, X; He, X; Ji, W; Du, S; Zhang, D; Zhu, D; Gao, H (2005). "Stable, Reproducible Nanorecording on Rotaxane Thin Films". J. Am. Chem. Soc. 127 (44): 15338–15339. doi:10.1021/ja054836j. PMID 16262375.
- ^ Yerin, Andrey; Wilks, Edward S.; Moss, Gerard P.; Harada, Akira (2008). "Nomenclature for Rotaxanes and Pseudorotaxanes (IUPAC Recommendations 2008)". Pure and Applied Chemistry. 80 (9): 2041–2068. doi:10.1351/pac200880092041.
